“I don’t have access to our Product managers’ data, so I have no choice but to harass them by mail when certificate renewals hit.”
“We have a very low non-conformity occurrence rate, but they drain too much bandwidth to solve when they happen.”
“We run frequent audits on our sites but lack the tools to analyze them.”
Product quality management is a top priority concern
Do your teams have separate data and document management solutions? Do you have access to an overview of your regulatory documents and their status? Have you been failed several times to submit a certificate renewal in time?
Quality management discrepancies can have unfortunate consequences on the brand’s financial and image capitals. Some brands never recover from a scandal, losing consumers’ trust and ruining their image. Even if that is an extreme case scenario, quality mismanagement will lead to an extended time-to-market and long-term losses of profitability.






Quality management covers several challenges
To avoid that, product quality control management must be enforced collaboratively, and on a regular basis.
- All product information and documents related to New Product Development (NPD) projects must be stored in a central database that everyone involved can easily and safely access and use.
- All the contributors of the new product development chain must be able to communicate seamlessly with each other, including external partners such as raw material suppliers.
- Organizations must comply with various and complex regulations and standards: USDA, FDA, GMP (Good Manufacturing Practices), ISO (ISO 9001, ISO 22000, ISO 22716, etc.), INCO, etc.
- Quality control plans need to be accurately operated, tracked, recorded, and updated. Brands must be able to react quickly in case of any safety alert, non-compliance occurrence, or consumer complaint. This includes being able to automatically trigger the adapted workflows and ensure their timely achievement.
Aptean Food and Beverage PLM Lascom Edition as your food and cosmetic product quality management software
Following the core concepts of the Product Lifecycle Management (PLM) technology, we see centralizing the information and consolidating the processes as the key factors to a responsive and rigorous management of quality issues.
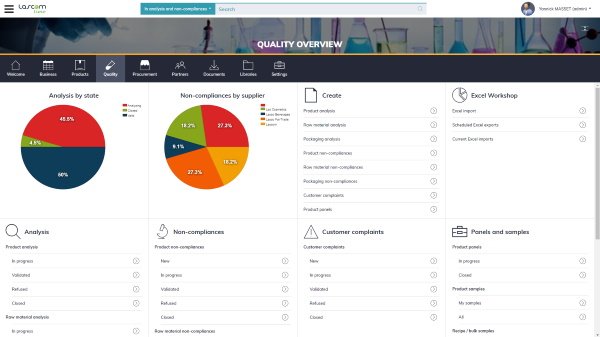
Aptean PLM Lascom Edition offers a comprehensive set of features all the teams involved in new product development projects. It comprises a consolidated Quality Management module designed to answer the challenges of non-conformity and compliance management, analysis management, and panel management.
Aptean PLM Lascom Edition provides you a collaborative environment to gather both internal and external quality-related items: certification documents, panel results, customer complaints, etc. Your teams can enrich them with photos, documents, or user comments for instance, and trigger their customized workflow to tackle the issue as quickly as possible.
Quality is not a formal post development check; it must be seamlessly integrated to every step of your design chain. This is what Aptean PLM Lascom Edition offers.


Our PLM offer for Food & Beverage product manufacturers!





