“Once information is captured, I can not reuse it for new projects.”
« I spent too much time gathering and formatting specifications. »
“We have too many validation protocols and it slows us down.”
“I have to chase and compile data before I can actually work.”
Do you keep receiving product and packaging specifications by email and then have to manually store them in your database? Is your database an Excel sheet saved in a cloud everyone can access and modify? Does every supplier send you information in different formats?
You may have worked this way for a long time, but data is the new “El Dorado”. You can’t deal with it like you did 10 years ago, and it was already a strategic mistake even then.
You need reliable methods and tools to centralize and double-check all the information required for your new product development projects.


Solution
Consolidate data before building product specifications
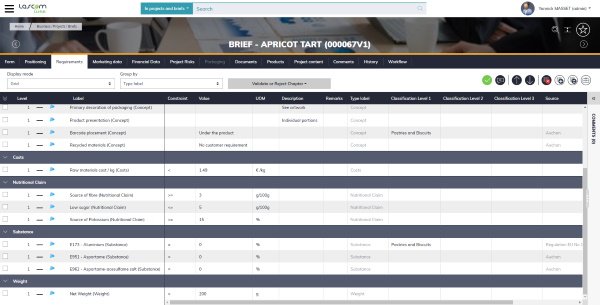
Aptean PLM Lascom Edition provides a unique repository for paperless new product development (NPD) related information: such as raw material and packaging characteristics, mandatory files, suppliers’ details, etc. You define every item using a form with various fields to be completed, either free-format or from lists of existing options including classifications, nutrients, ingredients, physical-chemical criteria, and many more.
All specifications are stored to build a healthy and logical database. Data formats, dependency relationships and criticality levels are all met, and then positioned in the NPD process. This makes finding and using data in later steps intuitive, efficient, and secure.

“Once information is captured, I can not reuse it for new projects.”
Have you ever been asked to develop a range of products from one you created two years ago? Has your main supplier unexpectedly replaced half of their catalog?
Even after you have finished developing a product, its data lives on. You could make us of a new regulation by using the formulation base of a currently marketed product. Or you might need to make mass replacements in your raw materials library and update regulatory documents, such as the Product Information File (PIF), accordingly. Information continuity does not end with the marketing of the product.
You need to be able to reuse work you have done in the past, seamlessly operate wide-ranging changes in your databases and ensure data accuracy even after it has been approved.
Solution
Secure critical tasks
Aptean PLM Lascom Edition comes with a comprehensive set of functional modules such as the formulation tool. This automatically calculates the product’s nutritional facts panel, ingredients statement, allergen list, claims and so on. It then uses this to generate the product label in several languages, and in complete compliance with your marketing and regulatory requirements.
This considerably secures and speeds up time-consuming steps such as formulation, prototype simulation, generating specifications or regulatory compliance approval. Significant direct advantages in the form of a safer final product and a much shorter time-to-market are the result.
The PLM stores all the information related to your products and packaging and keeps track of every modification. Your R&D team can consequently start to formulate by taking any finished product or prototype that already exists as a start point. When altering a raw material specification, you can also assess all the products might be affected and update their specifications accordingly.
“We have too many validation protocols and it slows us down.”
Are you sure you made no errors when compiling your finished product’s specifications? Did you forget anyone when sending them for approval?
Successfully gathering data is only the first step. Whatever your role – marketing, research and development, procurement, regulatory affairs, etc. – you inevitably make changes to this data. Amendments, deletions, additions, updates, you name it. There is always the risk of introducing error, particularly during repetitive and laborious tasks. When you are done, you need to send the data to your team- or external stakeholders- without it being distorted.
You need secured processes and communication channels as well as automated critical tasks to eradicate data-handling errors.

Solution
Collaborate efficiently with your teams, suppliers and partners
Companies have to orchestrate both their internal processes and their relationships with external stakeholders: suppliers, laboratories, creative agencies… Aptean PLM Lascom Edition safely secures all these interactions.
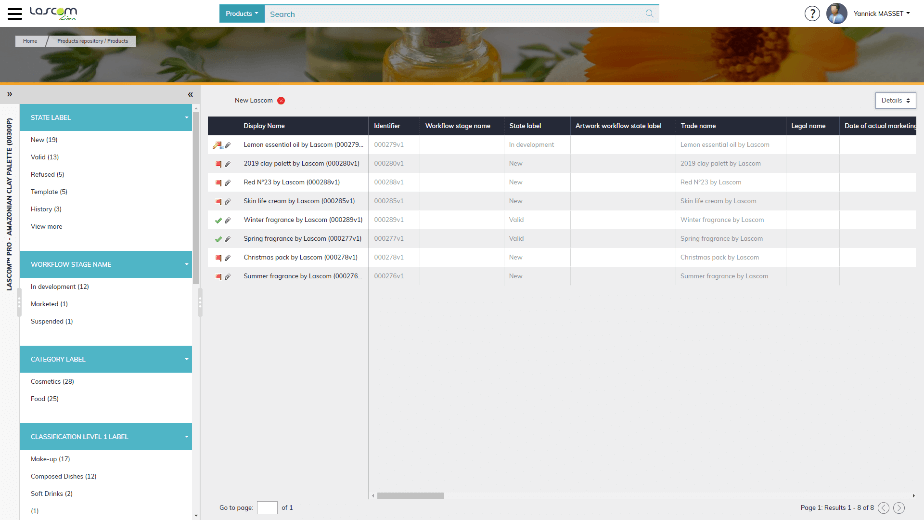
First, every external stakeholder can access your repository with the supplier portal, which is a strictly controlled administration. They can capture various information such as raw material or packaging specifications, certificates, plant locations, company details and so on. Product specifications can also be imported via automatic uploads of Excel sheets.
Second, its workflow engine lets you model all your NPD processes: task sequences, critical steps, delays, validators… It also sends reminders and alerts to concerned users and creates project management reports.
Suppliers can provide the raw material and packaging specifications directly with the dedicated portal or via Excel templates. The Research & Development and Quality team can retrieve this information to formulate the product, simulate different prototypes, generate the batch sheets and the finished product’s specifications. They can easily send these documents to internal validators, production plants and the customer. This workflow ensures that all who are involved verify and approve that all required information and documents are accurate.
Our PLM offer for Food & Beverage product manufacturers!

Product specification management:
what our customers say!
Product specification management:
what matters
Repository
The PLM technology core element is to provide a unique and central place to manage all product, project and packaging related specifications. This ensures a single version of the truth.
Supplier portal
The PLM allows your suppliers and partners to enrich your database in a safeguarded environment. This reduces the time necessary for specification inputs and the occurrences of manipulation errors.
Automation
The PLM automatically calculates numerous key values for product development and generates required contents and documents.
Workflow engine
The PLM puts the data in motion through predefined and secured processes. This streamlines the whole flow of information.
ERP interface
The PLM can interface with various other solutions such as ERPs. This enables a seamless transition from product design to production thanks to ERP interfaces.
Solution brief
Product specification management